Glaucoma: what my doctors never told me that could have saved my vision
By Steve Kirsch
If you are being treated for glaucoma by a glaucoma specialist, yet still losing your vision, the information on this page might save your eyesight.
As of 10/2014, my visual fields reversed and now some of the black area is gone. Doctor said, “that’s VERY rare." What I did that caused this is I went on a ketogenic diet. Now have super low blood pressure and my blood sugar is now under control (I had been diabetic for 7 years and nobody knew). I think my high blood sugar caused by my diabetes was contributing to my glaucoma, but I cannot prove this.
This page describes the vision loss in my left eye due to glaucoma. Thanks to a dumb treatment regimen recommended by a a respected glaucoma specialist, I have lost almost 1/4 of the vision in my left eye and it has now also affected on my central vision (known as "fixation"). This was avoidable if you read the information on this page.
Unfortunately, if you rely on what most ophthalmologist will tell you and the consumer information currently being distributed by well-intentioned organizations (such as the highly Glaucoma Research Foundation), you will remain in the dark, and the same thing that happened to me could be happening to you right now if you have glaucoma that is not under control.
Short story
Here's the synopsis of what I learned (and unfortunately, you will RARELY find anyone who will tell you all this):
- If you have visual field loss, you should take serious action IMMEDIATELY to stop it from progressing. By the time you get loss in a visual fields test, it means you've already lost a shitload of vision PERMANENTLY and it is now so bad it is starting to show up in tests. If you don't believe me, get an OCT done and look at the RNFL heat map. It will show massive vision loss. At this early stage, you can use the visual fields and the OCT RNFL heat map to see your progress. If you continue to lose vision, the RNFL will eventually become so sparse that the only way to track the little vision you have left is the visual fields test.
- All vision loss test are noisy. So you have to be careful in interpreting them, but they give you an objective measure of where you are. Take two OCTs one right after the other and compare them and even though they are taken at the same time, they will look different... it will look like you just either lost or regained vision! So you have to be careful because your eyes typically are getting worse at a slow rate and it's going to be really hard to see such small changes over time because the tests themselves are noisy. Even the latest OCTs that compensate for motion (and I've tried them all) are still noisy so small changes in your vision are hard to track. Tracking your average RNFL thickness in each quadrant seems to be a less noisy measure of loss since these are relatively stable between scans done at the same time. The best way to verify stability of the testing device is on the machines measuring you so try two scans right after the other and you'll get an appreciation for the noise level. Generally, if you do 4 tests and take the average RNFL of all quadrants (usually the top number on the printouts), you will get a reasonably stable value that you can compare with other scans. DO NOT JUST TAKE ONE SCAN PER VISIT. That is way to too noisy. It's like taking blood glucose measurements...any individual test is within 10% of the mean so by taking several measurements, you get the true mean value. So you'll find the same thing with RNFL thickness measurements. About 4 measurements per visit will reduce the statistical noise.
- Even if you are losing your vision at a very rapid pace, it may take 3 months or more for this to overcome the measurement noise of the instruments. That is a big reason you should move aggressively when you see any vision loss... you'll try things and won't know for months whether it worked or not, and in the meantime you are permanently losing vision.
- Your objective is to stop the vision loss. The only two proven ways to do this are: 1) lower your eye pressure (IOP) and 2) avoid like the plague any drugs that lower your blood pressure (such as Timolol and Combigan both of which were prescribed to me by supposedly competent physicians).
- Your doctor only measures your IOP during daylight hours. He typically just takes a single measurement and has no idea how your pressure fluctuates over a 24 hour cycle. Lots of people have dramatically higher IOP at night (around 5am in the morning is peak for many people). My IOP doubled at 5am from the value taken in the doctors office at 10am. This is likely the cause of vision loss that is "inexplicable" but nobody can really know for sure because we have no way to measure vision loss on small time scales (of hours).
- Unfortunately, it is not so easy for people to accurately measure IOP at night. I did a sleep study at UCSD to find out. If you want to be safe, assume your IOP doubles at night so even though you think it is really low that's because you are getting reading from your doc in the daytime. Most docs never tell you what happens at night.
- You want to ramp up the treatment to stop the vision loss ASAP. I'd go aggressively on the eye drops since that is easy, low cost and low risk. Then if you stop progressing you can back off. This is better than going less aggressively and finding you are still permanently losing vision. There is really no disadvantage to starting aggressively on drops. Unfortunately my original glaucoma doc took a "let's try one drug" approach which is simply terrible advice in my opinion because there is no real downside in the triple drug combo that I'm now on (other than slight inconvenience of doing drops 3x/day). My original doc followed the typical "standard of care." But there are way too many examples of how the traditional standard of care is simply wrong and stupid. Look at diabetes. The standard of care is horrible. It will take years for the ADA to change its recommendations. And look at the American diet. Most of the stuff they've been telling you for decades about fat, carbs, etc. is just plain wrong. Only decades later is this starting to get serious attention (cover story of Time on "butter" in June 2014).
- NEVER EVER assume that the medical standard of care is what you want. This stuff, like the medical profession in general, changes incredibly slowly. It is really hard to change people's beliefs once they believe something. I have diabetes and I can tell you that the standard of care is absolutely horrible and will make your diabetes worse.
- Check for diabetes. I had diabetes for years and none of my doctors picked it up despite the fact I had blood tests every few months for my cancer. It's simple to test. If you ever have a random blood sugar above 140 mg/dl, you likely have diabetes. Ask a diabetic for a test strip and a new needle to prick your finger and look at your blood sugar 1 hour after a meal containing about 50g of refined carbs (drinking apple, orange, grape, or other fruit juice is a good way to do this test). For normal people, their blood sugar will move hardly at all. If you are above 140mg/dl, you're likely in trouble. That's the simplest, cheapest way to test for diabetes. You can also have a blood test and look at A1c to see if it is below 5.7%. If you don't pass, you should get on a low carb, high fat diet as soon as possible (with about 70gms of protein/day) to stop your diabetes from getting worse and keep your blood sugar under control. I'd recommend this diet to everyone. The more carbs you consume, the more likely you will be to develop diabetes and then you REALLY need to cut back on your carbs . So a modest change in eating habits today by reducing your carb intake to 50gms/day or less, the more likely you will be to avoid getting diabetes.
- Check your blood pressure especially at night. If it is low, this could be your problem. In general, you want your diastolic BP - IOP >50. If it is less than this, you will likely still have vision problems where IOP is your worst case IOP (which could be at 4am). My BP is 75 and my IOP at 5am is around 25 (even though it is 12 in the daytime), so 75-25 (at 5am) so I'm just above the 50 danger mark. Note that my daytime IOP can go as low as 10 to 12, but I think it is might high nighttime IOP that is the problem.
- Starting with eyedrops is the safest and easiest approach. The best medications are Alphagan-P (morning and afternoon, Azopt (3X/day), and Xalatan (at bedtime). There are lots of reasons for this combo and the timing. Alphagan lowers your blood pressure, so don't use it at night. And it doesn't work at night either. Azopt is a great drug and works at night. Xalatan also works at night and into the next day, but has strongest effect within hours after you take it so that's why you want to take it at night because your highest eye pressure will likely be at 4 or 5am in the morning. Basically, you want to time these drugs for peak effectiveness around 4am to 6am. So that's why the regimen.
- Never assume that just because the FDA approved a drug that it will be helpful. I had a friend on Timolol who was losing her vision. That drug lowered her blood pressure which increases your risk of vision loss. She switched to Lumigan (which doesn't lower your blood pressure) and no more vision loss. I can make the same argument about Januvia for diabetes...it is very popularly prescribed by physicians, yet there is strong evidence it causes irreversible damage to your pancreas.
- If the three drop regimen doesn't stop your progression, then SLT surgery is really quick and easy and you can try that.
- If that doesn't do the trick, then you can try Trabectome surgery if you can find someone who is SKILLED at it. This is pretty effective and a lot less problematic than trabeculectomy surgery which is the surgery of last resort due to risk and complications.
- I use a Reichert 7cr at home that I spent about $7,000 to buy so I could make sure my IOP is under control 24x7 so i can get up and test my eye pressure at night. But that is likely overkill since the treatment protocol is the same even if you don't have a home tonometer. The Reichert uses "air puff" to measure your IOP and unlike the gold-standard Goldmann tonometer, the Reichert corrects properly for cornea thickness (it isn't the thickness of the cornea that matters but the elasticity and the Reichert measures this). Some papers claim the Reichert is the more true/accurate measure of IOP. I don't know enough to comment on that, but the papers I read were pretty convincing.
- Bob Weinreb at UCSD is the smartest guy I know in the glaucoma space.
Longer story
Because I failed a visual fields test, my optometrist suspected I had glaucoma, and referred me to a respected glaucoma specialist. After five years under his care, I lost nearly 1/4 of my vision in my left eye. I went to get a second opinion and discovered two things that really surprised me: 1) I was given drugs that not only didn't help, but probably made the problem worse and 2) I was recommend a surgery that was much more risky that safer alternatives.
Why did this happen?
My ophthalmologist:
- never told me that eye pressures can rise dramatically outside of office hours and that he lacked a complete picture of what is going on,
- never told me that there are inexpensive ways (such as using an iCare tonometer) available for me to measure my eye pressure over a 24 hour period (and there of course wasn't an iCare tonometer I could borrow for a night to find out what was happening)
- never had any clue what my eye pressures were outside of office hours,
- observed that I was progressing rapidly, yet my "checked in office" eye pressures were "normal", but yet never suggested that the problem might be that my IOP was peaking outside of office hours
- never told me that the drugs he was giving me were not effective at night (I later learned that my eye pressure can rise nearly 3 fold to over 30 when I am asleep) and might increase the rate of progression because it might lower my blood pressure. His drug regimen, in my case, according to one of the world's best glaucoma doctors, was worse than giving me a placebo because Timolol can lower your blood pressure by 15 points or more (which is huge because it impacts ocular perfusion pressure).
- never measured my blood pressure, despite putting me on Timolol
- never suggested that I should take a carbonic anhydrase inhibitor, despite the fact that many experts believe that the best option for controlling IOP are prostaglandin analogs and carbonic anhydrase inhibitors (See The question of IOP).
- never mentioned to me that it may not just be the high IOP that is bad, but also having low blood pressure and having a large diurnal IOP fluctuation (normal is 3, over 4 is a red flag, and mine is over 18),
- never mentioned that there is a surgery called Trabectome that is low risk with an 84% overall success rate and a mean IOP reduction of 40% that would be appropriate in my case to control the IOP in hopes of stopping the progression. Instead, he suggested the more risky trabeculectomy.
Had I been given this information and effective drugs to match my IOP pressure curve, I might not have lost the vision I did. Am I upset about the treatment I received? Absolutely. I totally trusted this doctor due to the opinions of other doctors. For something as serious as your vision, it pays to do the research as soon as possible.
Hypothesis
I have a few ideas for what might be causing my vision loss. It could very well be combination of factors, rather than a single factor:
- High IOP at night (as high as 29)
- Low blood pressure at night (low 70s)
- Sleeping position putting pressure on the eye (if face up is baseline, face down is +4, and on your side is +2 in the lower eye)
- Sleep apnea/CPAP mask that has high re-breathing amount leading to lower O2 saturation. If I don't "seal" the mask perfectly, the air I breathe feels "fresher."
- Waldenstrom's (may or may not impact; the number of WM patients with glaucoma I don't think is above average)
- I have undiagnosed diabetes that spikes my blood sugar to nearly 250 (I just found this out in May 2014, but I have been having it for years)
- Low CSF (you can have this without headaches). See http://www.ncbi.nlm.nih.gov/pubmed/24736050
- Autoimmune disease attacking my RGCs (Marty Wax's favorite theory)
Marty Wax thinks it is anti-retinal ganglion cell antibodies that are killing my vision related to my WM. So the following approach is suggested:
- Have Gulgun Tezel, Jeff Goldberg, and/or Ben Barres test my blood to see if retinal ganglion cells survive. This is the shotgun approach to see if there is anything in my blood that might be causing premature cell death of my retinal cells.
- Have my blood analyzed by Alan Pestronk. This can be done remotely, without a physical office visit.
Longer version of my story
- At first signs of Glaucoma, I was referred to a well respected glaucoma specialist in my area
- The specialist never measured my pressures over a 24 hour cycle to determine the proper medications to prescribe and to determine the severity of the problem. He never informed me that this was possible or recommended or that I could buy an iCare or Reichert tonometer and do it myself at home.
- The specialist prescribed medications, then halted them for 1.5 years, then prescribed medications that were known in the literature to have no impact at night which is when I have my highest pressures (over 30). Furthermore, the drug combination I was prescribed (Combigan) also lowers blood pressure which can accelerate the damage. So what I was given was worse than a placebo...in all likelihood it actually accelerated the damage!
- Virtually all of my vision loss was during the times that I was only "no drug" or "drugs that only work in the daytime and lower your blood pressure" (Combigan) regimens (see fields test result below). My IOP was never measured except during office visits. The doctor had no clue my IOP peaked in early morning when the drugs he prescribed would have little to no effect.
- I was then told I needed major eye surgery (trabeculectomy) if I wanted to save my vision.
- I decided to get a second opinion.
What happened when I saw one of the world's best glaucoma doctors (Weinreb at UCSD)
- I was immediately switched to a drug regimen using a two drug combination (Prostaglandin analog and carbonic anhydrase inhibitor) each of which is shown in peer reviewed studies to be effective at night and would be providing the maximum impact.
- I was sent to the sleep lab to have a 24 hour IOP measurement. I discovered that my IOP was low during waking hours, and peaked at over 30 overnight even though I was now taking two different "best in class" drugs to lower pressure at night. I can't imagine what my pressure would be on no medication or medications that only work in the daytime
- Because even when I was using drugs that worked at night my IOP was still too high and my central vision was now impacted, he recommended trabectome surgery, which is far less risky and life changing than the only surgical option I was offered by my initial physician
- I had the surgery done and my eye pressure after the operation was 9 in the operated (left) eye.
My best advice to preserve your eyesight no matter who you are is this: Get a Cirrus OCT once a year and take a look at the RNFL thickness map and track this over time and look for changes in the color map. You'll see if you have a problem way before you start losing your eyesight so you can stop it before it permanently takes your vision. By the time your vision loss shows up on a "visual fields" test, that vision is already permanently gone. So if you want the best chance to protect your vision for the rest of your life, get an RNFL thickness map on an annual basis and take any progression very seriously.
Six things everyone should know (that you probably are not already aware of)
-
Most people have peak IOP early morning while in bed. So the IOP your physician measures (during office hours) can be largely irrelevant. While we know that lowering IOP slows progression, we cannot prove yet that most of the RNFL damage is being done at night. However, this is the most logical conclusion.
-
That peak IOP can be huge. I am 12.4 during the evening and 30.5 12 hours later. Make sure you know by using an Icare or Reichert tonometer at home (e.g., buy or get one on loan from your ophthalmologist).
-
The risk of disease progression within 5 years was six times higher for patients who had a diurnal IOP range of 5.4 mm Hg than for those with a diurnal IOP range of 3.1 mm Hg. So you want to control your peak pressure to keep the range down.
-
Drugs do NOT work evenly over 24 hours. Some drugs have virtually no impact during the night (when pressure is highest). Some drugs work for days, others for hours. Know the 24 hour effectiveness profile of each of your medications. You may discover that your drugs do virtually nothing at night.
-
There is a bunch of data suggesting that OPP is more important than IOP. This can't be proved yet. But if you believe it, then drugs that lower your blood pressure (such as Timolol) are really bad for you. If you are not sure, it may be much safer to simply to avoid drugs that lower your blood pressure.
-
Get regular OCT measurements and look at the RNFL heatmap. This will tell you if you are losing your vision. Take steps to stop it before it starts impacting your visual fields test. Your RNFL losses are permanent so the sooner you stop/control your progression, the better. Try different meds to stop. If that doesn't, then do a surgery.
-
Trabectome, done by a skilled surgeon, is effective 84% of the time and is much safer than trabeculectomy. 40% drop in IOP is typical.
-
The average person (without glaucoma) loses about 1-2 microns in RNFL thickness in a decade. People with glaucoma can lose >1 micron/month. So this is a pretty good measure of progression.
Suggestions I have for glaucoma specialists
Had my doctor followed these recommendations, I would likely not have suffered any noticeable vision loss.
-
It's fine try different medications, but try each medication for a week, then look at IOP at various times against the target IOP range objective, and if it is not achieved, try different drugs (adding or switching). Do not leave someone on a medication without confirming that the peak IOP was reduced by the medications. Once all medication combinations have failed to reduce IOP, clearly explain that the alternatives of surgery vs. further vision loss.
-
Offer to loan out a self tonometer (such as the iCare) for free to patients
-
Offer to explain why each drug was prescribed for the patient based on the IOP profile and the drug diurnal profile.
-
If prescribing any drug that can make the glaucoma worse, such as Timolol, clearly explain this to the patient and the rationale for prescribing this.
-
If prescribing a drug regimen (such as mono therapy Combigan) that do not work at night to address high IOP at night, clearly explain to the patient that there is no scientific basis to believe that that such a regimen would be effective.
-
Tell your patients all the viable surgical options, even the ones you do not do, e.g., trabectome has an 84% success rate (yet was never offered as an option for me by 2 doctors I saw).
-
All patients should be given a Glaucoma Information Sheet that covers these points:
-
Your IOP may be much different at night. To ensure the best outcome, we recommend you use a tonometer at home and get a 24 hour profile.
-
Once vision loss shows up in fields test, that is permanent functional vision loss. If we are effectively treating you, the rate of loss in a fields test should be very minimal.
-
The best way we have right now to objectively track progression is the OCT RNFL thickness map. Glaucoma will show up in the OCT far sooner than it will show up in the fields test so if we are effectively treating you, you shouldn't see much variation in this map. So treating you very early and stopping progression as seen on the OCT can prevent any functional vision loss and it gives us the most amount of time to study your rate of progression and to try different drugs.
-
All of the drugs we give you have a very short half life. So after you've been taking a set of drugs for a few days, we can measure your IOP and see if we are controlling both the peaks and the range. If not, we should switch drugs and/or add drugs until we bring your pressure under control
-
It is advisable for the IOP to be relatively stable (within 3mmHg over a 24 hour cycle). Huge swings, such as from 12 to 30, mean we have not done our job of controlling your glaucoma
-
Different drugs have different diurnal profiles. Our job is to look at your IOP profile and match the drug effectiveness profile to your IOP profile.
-
There are three things we can do to reduce chance of progression: lower your peak IOP, lower the dirurnal IOP variation, make sure you are not on a drug that might lower your blood pressure.
-
Certain glaucoma medications, such as beta blockers, might make your glaucoma worse. In particular, beta blockers can lower your blood pressure and may increase your risk of progression. If a beta blocker is prescribed, we should carefully measure the impact on your blood pressure to make sure it is not being lowered.
-
My risk factors
The key to managing glaucoma is to eliminate as many risk factors as you can
and see if
-
My IOP with aggressive pressure drugs has been as high as 29 at 5:30am. Only one doctor figured this out: Weinreb.
-
My diastolic pressure was professionally measured during the day to be as low as 58. That is a huge risk factor. So my DPP has been <<50 at times due to high IOP + low diastolic blood pressure.
-
Waldenstrom's macroglobulinemia, but currently under control with LBH-589 which is an HDAC inhibitor (see Kirsch Waldenstrom's Macroglobulinemia Diary). I don't know if the LBH-589 lowers blood pressure, but that is one of the first things to check to see if any medication lowers blood pressure.
-
sleep apnea (I use CPAP every night). CPAP in general raises IOP by a few points. This could have put me over the edge. So this may be the difference right there. But only 1 doctor knew this!!
-
The way I sleep (on side) perhaps has been suggested. Pressure increases on the side you sleep on.
-
My Dad had diabetes around my age
-
Drance heme seen before I progressed
Gülgün Tezel, M.D.: we have already run some analysis (called SDS-PAGE of retinal proteins followed by immunoblotting to probe with your blood) and detected some antibodies in your serum samples which reacted to some retinal proteins. We will now run more advanced analysis (called mass spectrometry) to identify what specific retinal proteins your blood reacted to. I expect that this analysis may take couple more weeks. We can not simply tell whether these serum antibodies are normal or pathogenic, but in your case, we can determine whether they exhibit an increase or decrease in their titer over time with any correlation to your disease progression. I bet most of these antibodies can be found in disease-free people as well; however, in some predisposed individuals (like yourself) they may be the last drop that spills the cup and contribute to disease progression. In our ongoing experimental studies using animal models, we hope to better understand when/why/how they might be pathogenic.
Weinreb's diagnosis: OAG, i.e., open angle glaucoma. Your presentation is typical for open angle glaucoma - progressive bilateral optic neuropathy with excavation, beta-zone parapapillary atrophy and progressive loss of retinal nerve fibers over many months. Moreover, your optic nerve does not have pallor exrcept in areas corresponding to loss of retinal nerve fibers. As we discussed, there is little that is certain in medicine. Rather we make decisions based on likelihoods. In my opinion, it is highly likely (VERY highly) that you have open angle glaucoma. Susceptibility of optic nerve to damage enhanced by possible risk factors including blood viscosity/oxygenation, sleep apnea/CPAP.
Hanley's diagnosis: low tension glaucoma aka normal tension glaucoma (NTG) from the data you showed me. does it matter yes and no. NTG is in general harder to manage and in people under 60 one thinks of other things that contribute i.e. Waldenstrom's.
Wax's diagnosis: auto-immune glaucoma since I have WM and people who are prone to diseases of the immune system are more likely to develop other autoimmune diseases. Gülgün Tezel can test for this using blood draw. Tests for anti-retinal antibodies including those against HSP60 and HSP27 which kill RGCs. If I have anti-retinal antibodies, treatment options are: (1) plasmapheresis (expensive and insurance won't cover, but lasts for 3 months), (2) chemo drugs like cytoxan (but horrible side effects), and (3) drugs such as novel agents such as Rituxan, Velcade, etc. (perhaps even TNF alpha inhibitors such as Enbrel, Remicade or Humira which may be useful against WM). Franz Grus in Germany can also do this test.
Note: Normal pressure glaucoma (often inappropriately called "low tension" or "normal tension" glaucoma) is actually a glaucoma with open angles, so it is technically a form of open angle glaucoma.
My game plan
-
Take full baselines measurements (sleep, triggerfish, OCTs, STARFISH study, Spectralis scan, etc). Weinreb is really the “man with all the toys” here. I was very impressed with his setup in San Diego and his team.
-
The sleep study showed that even after using Zioptan and Azopt, my pressures are way too high at night. So do trabectome for left eye. Risks: 16% of the time it doesn't work. It generally lowers pressure around 40%. You can get post operative pressure spike right afterwards (within 24 hours which is why they have you stay in the area overnight). May need express shunt or other measure if that happens
-
I would like to try different drops, e.g., Lumigan, to see if there is any measurable difference in IOP (absolute drop and/or daily variation). Weinreb says all the PG analogs are pretty much the same and the Lumigan data could be just marketing hype.
-
Get a Reichert 7CR to track pressure at home. Measure impact of dosing at different times, using different drugs, impact of exercise, CPAP, and stopping my cancer medications.
Separately, I think there is huge low hanging fruit here with OCT data if we can make it repeatable. If we can take it so two OCTs can "line up," then we can see progression in a month or less. The Spectralis OCT (with TruTrack) and the latest Cirrus 6.5 software (with FastTrac) align things better and should give more repeatable measurements that can be used for tracking progression. Unfortunately, I did 3 scans with the FastTrac, and they weren't repeatable. So nothing has achieved repeatability yet. Solve this and it is huge.
The Spectralis will only do a B-scan when the eye is looking at the dot. The Cirrus doesn't use a dot and instead takes the images and lines them up. Since your eye is moving and your IOP is constantly changing, the data is noisy. If you took repeated A-scans in the exact same area, some will match up, but not all. This is due to eye movement as well as surface thickness variation since the heart and blinking is constantly changing your eye pressure. Your eye can move a few microns or a pressure change can mess things up. You can't even do A-scans in the exact same place and get the same value. So the problem is simple: Once you can do two A-scans in the same spot and get the same data every time, that is the key breakthrough. Everyone agrees that such data be transformative. Because then you can measure progression on a small timescale to see what is working and what is not. You should use the full 2D RNFL thickness heatmap, and look for pattern changes, and not just the circle. This gives you the greatest sensitivity.
My opinion
The typical standard of care is: whoops, you failed visuals fields. Sorry that means your vision is gone forever in those area. try this medication protocol, come back in a month and if IOP is still high, we can try different drugs (even though the results of those medicines are mostly known in less than a week). However, we'll measure you during office hours rather than at 5:30am which is when a lot of people peak, so I'm sorry but the measurement may be not so meaningful. So hopefully we guessed right. Because if we didn't, it's surgery for you.
Based on what we know today, if you want the best chance of stopping vision loss, what we really should be doing is 24 hour IOP measurements and blood pressure measurements and keeping your IOP low and stable and keep the DPP (Diastolic – IOP) over 50 at all times.
Patients should also try different drops to see which are the most effective in their body.
Doing something as simple as switching from one eyedrop to another has been know to completely stop progression.
Doing an OCT regularly would also help. Standard practice is to wait for the vision to show in a fields test. But by then, it’s too late. You can see glaucoma coming a country mile away with an OCT and that is the time changes are MUCH easier to measure too because there is more “signal." Had I known what I know now, I would have done this.
I know there are practical reasons like insurance reimbursements and false positives that are the reasons why we do it the way we do today. This is a real shame.
Finally, my original doctor who came highly recommended to me, gave me Combigan. There is credible data showing that this was worse than a placebo (see notes on Combigan below). So if we can't educate doctors on the diurnal curve of the various medications, at least we should be able to educate the patients.
My current advisors:
- Dr. Andrew Iwach, glaucoma specialist (San Francisco, CA)
- Dr. Maureen Hanley (Boston, MA)
- Dr. Robert Weinreb, glaucoma specialist (San Diego, CA)
Other doctors I've consulted or plan to consult:
- Dr. Alon Harris at IU has ocular blood flow testing equipment.
- Dr. Cantor at IU is in year 4 of a 5 year study for OPP.
- Dr. Katz at Wills Eye Institute (Philadelphia)
Tests that you can do:
- sleep study w and w/o CPAP
- 24 hour IOP
- triggerfish
- STARFISH study
- blood test for genetic marker
- MRI thickness of optic nerve (CSF indicator)
- would like to look at raw spectralis OCT data and discuss in more detail with someone.
- ORA: Corneal hysteresis (CH) measurement. A low CH is correlated with progression.
Q&A
- Can I get a doppler imaging blood flow baseline measurement so I can quantify my OPP? Yes, but company couldn't get reimbursement so couldn't sell machine. They can do it on OCT. Not normal part of the test since no reimburse. Not usually part of research protocol either. Using optivue.
- How can we get repeatability of A-scans in the same place? When the eye is in the same position, the measurements are repeatable. But the problem is the eye moves. So is there a way to measure the RNFL map in a person, wait 60 seconds, and be able to align the second scan on top of the first and get sub-1um differences in thickness for most all spots?
- When you take new eye drops, how long does it normally take to get to IOP equilibrium (i.e., relatively repeatable diurnal IOP measurements)? 1 week? A few days? does it depend on the medication? A: generally a few days.
- If you understand your diurnal IOP variations, can you dose optimize your medications? i.e., is there a known diurnal efficacy curve for each medication? or is the diurnal curve for each med different in each patient? A: each med has a unique dirurnal curve and the curve on day 2 is different than on day 1 if it is a long lasting drug.
- We've known for over 20 years that home non-contact tonometry is accurate and beneficial. What is the current best instrument for this? the Reichert PT100 or the Pulsair intelliPuff Tonometer? The PT100 was found to be just as accurate as the Goldmann (.3mmHg mean difference) if you have pressures in the normal range (like me). Or something simple that uses contact like the Tono-Pen AVIA® Applanation Tonometer or the Perkins Mk3. Maureen likes the Perkins Mk3 and it is pretty cheap (about $1500). Or through the eyelid using a Diaton tonometer ($2750). Or the ICare One Personal tonometer . The iCare lets you take measurements yourself and attaches to a PC. No drops and no air! In the US you can only buy the original ICare for $3795. Fiteyes website recommends the Reichert 7CR ($8,200) and specifically now does not recommend the iCare One (although he likes the iCare Pro version which appears promising). The iCare One requires user skill, unlike the Reichert. iCare guy will come train you he's at 916-208-5784. The Reichert guy will hand deliver the unit for me to check out and he'll beat the $8,000 price. A: I went with the Reichert because it is fast, easy, no operator error, and very accurate.
- I've read that you can have progression in visual fields with no change in the OCT. is that really true? A: yes, but only if it is a central nervous system problem. So it is rarely true. .
- do the med frequencies make sense? e.g., morning and noon for Brimonidine ? A: yes, because Brimonidine doesn't work at night.
- is it better to space 3X/day drops 8 hours between doses? or morn, noon, evening which biases to waking hours? 8 hours between. 3 minutes. then wait 5 min. A: You should space it to keep your IOP stable, but generally 8 hours apart.
- is dose/response curve optimized for the 3 drugs? A: ideally, but I was given prescription before the sleep study.
- should i pinch nose or just don't blink? A: pinch your nose only for drugs which may have a systemic impact, e.g., brimonidine.
- 3 min close or 5 min? A: keep your eyes closed for 2 to 3 minutes.
- 5 minutes between instill even if close eyes for 5 minutes? A: give it at least 5 minutes after you open your eyes.
- electrical optic nerve stimulation? A: nobody has ever heard of it.
- repeatability of OCT measures and looking for fast changing areas? A: people are working on this.
Learnings from patients:
- Different drugs have different IOP lowering profiles. All drugs are not alike and you should test them on you (e.g., Lumigan is reported to work really well in some cases)
- Timolol put in your eyes may significantly lower your diastolic blood pressure in some people and that will adversely affect OPP which is an important factor. See Clinical Update: Glaucoma.
- Patients have reported a high correlation between OPP and the damage that was noted via tests. Making sure that OPP=diastolic - IOP is >50 is a good strategy for minimizing damage. So lower your IOP and do not do anything to lower your blood pressure is a good strategy.
-
Every patient with glaucoma is different and what worked for one patient doesn't necessary work for another. The fact that a patient's glaucoma has been stable for one year or more does not necessarily mean it will remain stable. Glaucoma progression is often not linear but can occur in spurts, so you need a longer time frame than one year to determine whether stability has truly been achieved.
-
A patient in Japan reported low CSF leakage causing vision loss in two cases from an unusual cause:
In the year 2000 I had survived a massive brain hemorrhage, after which I was implanted a vp shunt system. The pressure of the valve was originally set at 70mmH2O, but never changed . Last year, when I was desperately visiting doctors for help a neurosurgeon, looking at my head CT image, said there was nothing wrong with my head, but also said that maybe the VP-shunt system was overdraining...At that time I didn't give so much importance to those words but later my husband found the blog of a Japanese man who had a similar case and was almost completely blind (failure of drops, SLT, trabeculectomy...) and doctors recently found out that he had had a car accident in the past and the CSF kept on leaking all those years from his spinal cord, causing a lowering of the CSP...Now it seems he is not losing his sight anymore, thanks to a cure called "blood patch" to stop the fluid coming out.
Other learnings:
- the drug category that can cause or exacerbate glaucoma is steroids such as cortisone or prednisone. These are commonly used to treat many clinical conditions.
- To asses, use average RNFL thickness (early to moderate). use visual fields after that. Can use both.
- 90% to 95% of patients will stop progressing if we lower the pressure. If that doesn't work, you are in the 10% "hard to treat" category.
- IOP can vary greatly throughout the day and depends on what you are doing. So lifting weights can lower your IOP and thinking can raise it. You can see a 2:1 variance over the day. See http://www.fiteyes.com/Not-A-Typical-IOP-Day
- It isn't your absolute DOPP that matters, but your change from baseline (before you had glaucoma). So the DBP-IOP>50 is just a guide. Your actual threshold number may vary from this.
- For self measurement, the Reichert 7CR seems like a safe choice since it is easy to line you up for a perfect measurement. It also gives a confidence score that the measurement is accurate. The problem with the portable instruments is alignment. You get it wrong, you'll have inaccurate results. Even smart people who have been trained, often get it wrong with a portable instrument.
- Stress and the Valsava maneuver can increase IOP. Exercise seems to lower it, but weight lifting with heavy weights can raise it.
- Sleeping on one side can raise the IOP on the side you are sleeping on.
- Get a self tonometer like the Reichert 7CR or the Icare tonometer. Then you can easily try different eye medications and see the impact so you can optimize the dosing and timing.
- Tips for dosing experiments: From baseline, if you take a drug, it generally will reach maximum effectiveness after 6 to 8 hours. It will then slowly decline back to baseline over a period of 2 to 3 days. So you can stop your drugs for a few days, measure your baseline, then add a drug and see the effect in your body. Pay attention to how well it controls the IOP peaks since that is where the problems are. Also, re-measure after 4 to 6 weeks because a drug that appears to work at first in the first week or two, can have much lower effectiveness after you've been taking it for 4 weeks. If you are on a drug combination, you can try stopping and starting the drug and seeing the impact. You can also experiment with changing the time of day. CAUTION: your IOP has a diurnal cycle, so you need to make baseline measurements and then look at your deviation from the baseline diurnal curve, and not from a single measurement. One way to do this is to always take measurements at the very same time each day, e.g., when you get up in the morning which in many cases will be a high point.
- For many people, your highest IOP pressures are generally while you are asleep, or very early in the morning. So it is often the case that all the damage you are doing might be all done at night or first thing in the morning when your IOP is high and blood pressure is low.
- Agreement and reliability of candidate tonometers for measuring intraocular pressure reveals that Non contact tonometers are virtually the same accuracy as GAT...within 0.24mmHg (see Figure 28). Doctors still cling to the belief that non-contact tonometers aren't accurate though.
- Get your IOP and blood pressure measured over a 24 hour cycle so you can see where your peaks and valley are.
- The safe zone is when at all times your diastolic blood pressure minus your IOP is always >50.
- If you are progressing, test different drugs, even when your physician says that they are the same. Different people react differently. Studies show that Lumigan is the same as other drugs, but smart physicians know that Lumigan can be slightly more effective in some people and not others. So get those "samples" from you eye doctor and change every week and measure at the end of the week. Find out what works for you. This simple advice may be able to save you a surgery. This is not theory, it is fact based on real patients who switch drugs and their progression stops cold.
- If you are progressing fast, you can see a 3um average thickness delta in just 3 months. If you are normal, you'll lose about .2um or so per year. Patients can lose 7um/yr in the inferior region (as in the patient in the Zeiss datasheet on how to read an OCT). Note that this is a 3.5um overall loss, but by limiting the averaging to a narrower region (the inferior region), you can see much greater average change because the thickness change is NOT uniformly distributed in glaucoma (most people will lose the inferior region first; i lost my temporal inferior region first, for example).
- patients with low CH should undergo more careful surveillance in search for past VF progression. Lower CH could, therefore, be (1) a marker of increased susceptibility of the optic disc to glaucomatous damage, or (2) may be the result of glaucomatous damage itself.
- Minimum detectable change has to be 5um or more to "see" it on an OCT right now due to the noise in the positioning when they make the measurements. I agree with that. That's what I found on test data. The paper Test–retest variability in structural parameters measured with glaucoma imaging devices concludes "Although SD-OCT systems may be currently prevailing because of the volume of information provided and the relatively better test–retest variability, these systems need improvement in their test–retest variability measurement capabilities." This is really the key to fix this.
- When a neuron dies, it goes fast. A single neuron can go from alive to dead in 1 hour.
- when a neuron undergoes apoptosis (which is really fast and can happen over an hour), the axon dies at the same time and shrinks over 24 hours. Since average nerve fiber thickness is 80um and a dead eye is <40um, one can imagine that the shrinkage is close to 50% in living tissue. This is (by how much and over what period of time).
- Blood pressure seems to jump around, part of it is you blood pressure normally will vary quite a bit, but there can be variance in the skill of the person taking it. The computerized devices can sometimes exactly match the manual blood pressure readings, and other times I've seen it be 80 points off!
- Always talk to >1 doctor. I've learned different and very valuable things from each doctor.
- Both ends of blood pressure (too high systolic or too low diastolic) can damage the optic nerve.
- See your eye doctor regularly and get a visual fields every time at minimum. However, an OCT will reveal loss MUCH sooner than visual fields so I highly recommend this test for everyone. Once it shows up in a visual fields test, your vision is permanently lost.
- My field loss likely originated from an ischemic optic neuropathy from the Rituxan treatment of my blood cancer that caused an elevated IgM. The doctor at Stanford did not read the test results for 1 week. Had she read the test when it came in, I might not have had any vision loss. Learning: make sure your physician (or you) is reading your tests as soon as they come in.
- Take any visual field loss very seriously. If it progresses faster than "normal" take action immediately. Understand what those field tests mean, and keep a careful eye on how fast you are degrading. If you are degrading, try alternatives ASAP to HALT it. What happened with me is the doctor basically only wanted to do the trabeculectomy as a last resort which is right since it can cause cataracts and increase your eye pressure or lower it too low. So we tried drugs. But the drugs didn't lower the IOP that much, either because it was the wrong medications for me, I didn't keep my eyes closed long enough, I pinched my nose, or whatever. So he just stood by as I declined because the "cure" (the surgery) can make things worse. Had I changed the way I administered the drugs and taken the right drugs, this decline might have been avoided.
- Switching eye medications to drugs I take 3 times a day (see below) and simply keeping my eyes closed for a solid 5 minutes after each drop (no pinching like they tell you because that can make things worse) caused a dramatic and immediate difference in my IOP (from 16 to 12). This apparently is not well know since Dr. Tayeri still doesn't believe it.
- The SLT laser surgery further reduced my IOP from 12 to 10
- I have loss in just one quadrant of my left eye and it respects the quadrant boundary. This suggests it might not be glaucoma but could be neurological. The way to rule that out is an MRI of the eye and brain (orbital and brain MRI). Could be a problem In the chiasm behind the eye. But my symptoms were not consistent with a neurological cause, so I skipped the test.
- It is helpful to know my IOP and blood pressure during the night. This can be done either with a technician using a Perkins Mk3 hand-held tonometer. If the high IOP is happening at night, we can adjust medications, etc.
-
For my vision loss, doing a 10-2 field (76 points over 10 degrees) instead of 30-2 (76 points over 30 degrees) will give a clear indication the extent of the damage.
-
If your loss is only in one eye and one quadrant like mine, it may very well be neurological.
-
Unilateral glaucoma is usually trauma, artery related.
-
Dr Tayeri points out that the Shiley Eye Center in San Diego has a sleep lab where they did a lot of research on night time IOP. I don't know whether they offer clinical IOP measurement services to private patients, but it might be worth asking. The Shiley Center is also where they did the clinical trials on the triggerfish contact lens that measures IOP for 24 hours.
-
Patients who are more prone to low tension glaucoma (where the IOP is normal but you have vision loss) include patients who have systemic hypotension, anemia, cardiovascular problems, and sleep apnea. High serum viscosity (SV) also appears to be a risk factor.
- There are things that can possibly exacerbate the problem so you should
measure them to see where you are:
- High IgM/serum viscosity
- Low hematocrit (HCT), e.g., under 32
- Low diastolic blood pressure (50 or lower)
- Your medications (e.g., for cancer)
- CPAP (can increase IOP)
- Sleep (because IOP rises at night)
- Neurologic damage (such as a tumor in your brain or behind the eye impinging on the optic nerve)
- Sleep apnea is a risk factor low-tension glaucoma.
- How you sleep (head raised on pillow is best)
- To measure your IOP during the night, do not get up. Have a technician from a nursing home come to your home and use a hand-held Perkins Mk3 to measure eye pressure.
- To measure your blood pressure at night, wake up and take it while you are lying down. Low diastolic blood pressure is common in people with glaucoma.
- New medications may work at first, but may not continue to be effective over time.
- On my optic nerve photos one shows a Drance Heme. These are common for low tension glaucoma.
- Some research has shown a high incidence of glaucoma and worse glaucoma on the side you sleep on
- 568 (right eye) and 578 (left eye) cornea thickness which is thicker than normal, which indirectly means your IOP is a few points lower than what is measured
- Paper by Caulkins shows p38 inhibition can prevent distal axonopathy. In terms of p38 inhibitors for FDA approved indications, doramapimod is generically available, used for arthritis. But honestly, more work in animal models is needed to prove efficacy and safety for eyes -- and to test the FAR more potent experimental versions. However, right now we can ask on arthritis groups as well as glucoma groups to find patients who have both and see if anyone is taking doramapimod or any other p38 inhibitor (such as VX-702) for rheumatoid arthritis. The drug they used targets mostly the alpha isoform. According to Inhibition of p38: Has the Fat Lady Sung? (Feb 2009) more than 100 p38 MAPK inhibitors have been developed for the potential treatment of inflammatory and/or cardiovascular diseases, but the majority have been discontinued mainly due to undesirable side effects. VX-702, one of a series of second-generation, orally active p38 MAPK inhibitors, is under development by Vertex Pharmaceuticals Inc in collaboration with Kissei Pharmaceutical Co Ltd, for the potential treatment of inflammation, RA and cardiovascular diseases. Preliminary phase II results for the treatment of RA and ACS have been reported recently. One previously heralded agent, doramapimod (BIRB796), was thoughtfully studied in RA, Crohn’s disease, and psoriasis. It wasn't effective. Ultimately, development of this drug was terminated because of the development of liver function abnormalities. The implications of this are unfortunate because it means finding someone with arthritis and glaucoma who is taking a p38 systemic inhibitor is likely to yield nothing. So much for the "free" clinical trial data.
-
There are a number of very interesting and potentially effective neuroprotective agents for glaucoma that are being studied in our laboratories (eg CNTF, sirtuins, HDAC inhibitors, memantine). Very often what works in the laboratory, such as the Novartis compound tested by Calkins, is promising, but does not make it into clinical trials.
-
anti-oxidants like alpha lipoic acid might help.
- Ben Barres at Stanford: We are making the first inhibitors to a part of the immune system called the classical complement cascade, as my lab discovered that this pathway is killing synapses not only in the normal developing brain but gets reactivated early in neurodegenerative diseases that involve massive synapse loss including Alzheimers and glaucoma (in fact we published a paper not so long ago showing that inhibition of this pathway is very powerfully neuroprotective in a mouse model of glaucoma). We've already succeeded in making the first therapeutic monoclonal antibodies to the classical complement cascade and shown that these powerfully inhibit this cascade, both in mice and humans. And we have very exciting data showing that these antibodies completely block the disease process in two different neurological disease models involving the actual human pathological antibodies including neuromyelitis optica (a devastating form of MS) and Guillain-Barre Syndrome (an often severe form of peripheral neuropathy). We are testing them in several neurodegenerative disease models now and will soon have that data too (preliminarily that is looking very exciting). I strongly believe that this will be the first treatment that blocks neurodegeneration in Alzheimers disease which is our primary interest (as you know the recent trials with antibodies against amyloid have all failed in humans with Alzheimers).
- John Flanagan wrote an article "Both Sides Now: CSF and the Development of Glaucoma" that talks about the importance of OPP and CSF. The clinical relevance of these observations is limited by the invasive nature of CSF pressure measurement, which currently requires a lumbar puncture. Innovative research is being conducted to identify non-invasive ways to assess CSF pressure. One of these may be as simple as a measurement taken in the ear. Of course, manipulating CSF pressure may be inadvisable for reasons of systemic health. But identifying patients at risk of glaucoma and progression by assessing translaminar pressure differences may tell us which patients require the most aggressive IOP reduction to protect the optic nerve and prevent visual dysfunction in glaucoma.
- Neuro-ophthalmologists are rare: Kim Cockerham, Richard Imes, M.D. here in San Francisco, another specialist in Los Gatos (Dr. Iwach knows him), and Joyce Liao at Stanford.
- Kinase in Axotomy-Induced Apoptosis of Rat Retinal Ganglion Cells (2000) points out p38 inhibitors could be potentially useful for the treatment of optic nerve trauma and neurodegenerative diseases that affect RGCs, such as glaucoma. So very relevant in my case.
- Cerebrospinal fluid (CSF) pressure is lower in glaucoma patients; so is diastolic blood pressure taken at night in the supine position; and IOP is higher. OPP (which is blood pressure - IOP) has been measured in NTG patients is significantly lower than in the control group. SOURCE: Ramli N, Nurull BS, Hairi NN, Mimiwati Z. Low nocturnal ocular perfusion pressure as a risk factor for normal tension glaucoma. Prev Med. 2013;57 Suppl:S47–9. Trans-laminar pressure difference (IOP-CSF) is least favorable at night as well. (See Both Sides Now: CSF and the Development of Glaucoma, John Flanagan, Ph.D., M.C.Optom ).
- Eyeball transplantation is in the realm of possibility in the next 10 or more years. andy huberman (CFC2 group now), a previous postdoc in Barres' lab, now in his own lab at UCSD, has shown that retinal ganglion cells that are coaxed to grow down the optic nerve will then enter the brain and find their correct visual neuron targets and reconnect with them.
- My thoughts on the p38 is the same as my thoughts on trying to save neurons from dying. If the primary driver of the disease is synapse loss as our data strongly indicate, it makes the most sense to focus on blocking the synapse loss as all the other stuff (dendritic atrophy, neuron loss, and axonopathy) is secondary.
- If DPP=Diastolic -IOP>50, then you are normal and should not progress. No increased risk. Doesn't work for me though. I'm 138/79 and IOP of 16. If your DPP>50, you should not be progressing. If you are, you should look for the cause. However, if DPP <50 and you are seeing changes to your OCT, then lowering your pressure should help.
- Simply changing drops can be the difference between fast progression and no progression.
- Ganglion Cell Complex (GCC) is the collective term for the three retinal layers associated with ganglion cells: RNFL, ganglion cell layer and inner plexiform layer. GCC thought to be affected in early glaucoma. Optovue OCT has a report that measures this.
- Bosentan can protect against glaucoma damage. See http://www.ncbi.nlm.nih.gov/pubmed/18719081. These receptors are highly expressed by astrocytes and trigger reactive astrocytosis which is a gene expression change. We've found that reactive astrocytes start to secrete many complement proteins so I suspect that blocking these receptors is accomplishing much the same thing as complement cascade inhibition. See paper, "Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma" which says, "Similarly, inhibition of the endothelin system with bosentan, an endothelin receptor antagonist, was strongly protective against glaucomatous damage." What's cool is Bosentan is an FDA approved drug now on the market. However, Ben Barres points out, "Most drugs don't get across the blood brain barrier or into retina. This one probably doesnt. The side effects of blocking endothelin receptors in the eye and brain are not clear to me. I worry they could be substantial." Calkins wrote, "Hey! Yes, this could be dangerous in glaucoma, since bosentan is primarily in clinical trials right now for things like pulmonary hypertension, heart disease, kidney failure etc -- organs with lots of endothelial cells. The problem with such drugs is not the blood-brain/blood-retina barrier, but that they can "go systemic" through the conjunctiva of the eye. That's why the first generation of IOP-lowering drugs (b blockers) often caused cardiac infarction! "
- If you are taking eye drops and they aren't lowering your IOP, why are you taking them?
- According to David Calkins, Brimonidine is supposed to have a neuroprotective effect so even if the drops don't lower your IOP, they might be having positive impact.
- Ben Barres writes: I do think that imaging active complement activation at synapses would be an incredible way to image the degree of active glaucomatous degeneration, particularly as it could be imaged long before axons and neurons degenerated.
- In high pressure glaucoma, loss is steady, whereas in normal tension, loss is episodic and you can go years between losses. That suggests that things like lowered blood pressure might cause that (I notice my blood pressure is all over the place...see below).
-
DT-MRI and fMRI analysis often involves remapping 3D voxels onto a "standard brain"--I would think mapping one patient's scan onto their prior scan would be fairly trivial, even with angle change.
--even with gross remapping based on blood vessels and ignoring angle/tilt, because glaucoma progression is regional, the calculus for change should be able to filter to include only regional change.
--biology may be limiting, I don't think we know if there is biological multi-micron change in retinal tissue over short or long periods, for example with fluid shifts. This may need to be studied or validated at least.
--we also don't know in humans (let alone the variation among humans) the time course between axon dysfunction, axon injury in the optic nerve, and cell body loss in the retina. If the axons go first, which is suggested in humans and supported in animal models, I think axons should continue to receive some attention, both for measuring disease and promoting survival/regeneration. (Also axon regeneration is the major issue impeding whole eye transplant, which Larry Benowitz and Jeffrey Goldman are trying to assemble a consortium now to address directly.)
-
CPAP raises IOP....not sure by how much. Proven in non-glaucoma patients. Nobody has done a study for glaucoma patients.
-
Sleep apnea in glaucoma patients will actually lower IOP at night to match IOP of patients without glacoma.
-
Raises IOP: smoking, caffeine, supine position (by about 4mm plus or minus 2mm), swimming (because your head is out of the water and your body isn't), sleeping on that side will raise IOP on that side
-
Lowers IOP: exercise (for several hours), revasterol, marijuana (reportedly)
-
Current best measure of progression is RNFL thickness.
-
IOP actually fluctuates by about 1mm in sync with your heart beats.
-
Nothing beats the reichert 7CR for true IOP EXCEPT a direct measurement but that is against FDA rules. If you run the Reichert on a calibration eye, it will read exactly the same (within .1mmHg) every time. The only drawback is must be used in sitting position.
-
Reichert pneumatonometer is what they use at the sleep lab at UCSD because they need to measure you in the supine and sitting position. Pneumatonomter uses the average of IOP of 2-3 sec time period. Pneumatonomter uses the average of IOP of 2-3 sec time period, which should include 2-3 cyclic changes of IOP (along with the cardiovascular cycles). Cardiovascular cycle has a minimal effect on the pneumatonometer readings, but the breath has its effect.
-
In the Topcon DRI OCT, DRI stands for "deep range imaging". It is a spectral domain OCT but has a longer wavelength for deeper penetration. The 200 file is the one with the smaller squares - it is a 12x9mm scan (200 micron x 60 by 200 micron x 45). The 1000 file has the scan divided into 1000micron squares. The excel csv output file goes across first, then up and down.
-
The best way to adjust for alignment is to use the reference called the fovea-Bruch's membrane opening (BMO) axis. This will be available soon on the Spectralis.
-
What happens to your diurnal IOP after surgery? Nobody knows because they couldn't run a study because someone objected saying the data would be useless.
-
Does OPP matter? Makes sense, but hard to know. No solid data.
-
Drops. wait 5 to 10 minutes after you open your eyes between drops. Keep eyes closed and don't move eyes. You only need to pinch if you want to avoid systemic absorbtion (e.g., Brimonidine).
-
Spectralis OCT is cool. Does a B-scan, then waits for your eye to be in position before it does the next B scan. This is the eye tracking technology. So it takes longer, but the images are sharper.
-
if you want to line up your 2D RNFL heatmaps, you can use Gimp and use the transparency
-
Spectralis lacks 2D heatmap images. The topcon DRI can dump the superpixel data to a .csv file. That is cool. But you can't line up the data for similar scans on the same eye.
-
Dr. John Liu discovered that IOP might be way high at night because saw this in animals. So he had the idea to try in humans.
-
Different drugs have dramatically different diurnal curves. Some drug categories last for days, other drug types last for hours. See notes below. Also, uniformly drugs are only 50% effective at night and many drug types have NO effect at night.
-
Night (especially early morning) is likely where the most damage occurs. You are laying down (increase of 5mm) and you IOP is also elevated. I am 16 during the day, but 29 at 5:30am lying down!
-
In 50% of the people, one eye progresses first before the other eye goes.
-
Once you have damage, your rate of progression increases. Ugh!
-
Trabectome surgery can last for months or years. Be sure you are operated on by someone with >100 surgeries under their belt because by then they have refined their technique.
-
There is risk in any eye surgery. With trabeculectomy, there a lifetime risk of infection.
-
I think VERY few doctors actually know the shape of the effectiveness curves of the drugs that they are prescribing. If they did, they would be less likely to have prescribed Combigan to anyone.
-
IOP is the same whether you are exhaling or inhaling. Breathe normally when they take your IOP measurements. Holding breath does not necessarily make IOP go up on GAT. "Valsalva maneuvers" will. Patient position can play a role. With GAT, you tend to be more hunched over.
-
I wonder if it might be better to note the full IOP range at any given point in time rather than trying to compute an average. To compute the average should you take all 4 readings? or average the middle two? or average the two extremes?
-
David Calkins says p38 inhibitor basically makes it harder for cells to die when under pressure. But if cells are under too much pressure, the p38 won't save them. So the thought is that glaucoma makes cells more susceptible to pressure and the p38 inhibitor reverses that.
-
No way yet to determine if Trabectome would be effective before the surgery is done. We have been using anterior segment OCT to try to figure out in an individual patient where the site of resistance in the outflow pathway is located.
-
Doctors use GAT instead of Reichert for two reasons: compatibility and insurance re-imbursement code.
-
the 200x200 on the Cirrus report means it's 200 B scans (line scans), each with 200 A scans. It covers a 6mm2 area.
-
Triggerfish results proved that IOP really rises at night, just like the measurements confirmed.
-
Sleep apnea can make a glaucoma person and look like a normal person. When you add CPAP, pressures look like a glaucoma patient. So adding CPAP in general will raise your IOP.
-
when taking BP and IOP readings, maintain that state (sitting up or lying down) for 5 minutes to allow things to stabilize
-
BP readings should be within 5mmHg of each other, e.g., a good automated tester will be consistent to within 5mm on each reading.
-
All eyedrops can be safely stored in the refrigerator regardless of what they say on the label. Just don't put in freezer.
-
Glaucoma patients have lower BP at night but same BP during the daytime vs. normals.
-
FDA limits are 5 minutes per day per eye for laser scans (like OCT).
-
Uveoscleral outflow drops to nearly zero at night (reported by Dr. Sit at 2011 ARVO). That could explain why some medications work better during the day than at night.
-
You want to minimize the IOP fluctuation. Mine is 18mmHg. Red flag at 4. See The question of IOP which says:
Dr. Varma calculates the difference between the highest IOP reading and the lowest IOP reading (taken during office visits at any time of day). He considers any variation higher than 4 mmHg—and, especially, higher than 6 mmHg—to be a red flag.
- The more advanced the glaucoma, the more narrow the range of variation/fluctuation should be. "Long-acting drugs are better in controlling fluctuation, such as all prostaglandins and Cosopt," Dr. Asrani says. "The range with these eye drops is within the range of a normal eye fluctuation." He notes that a combination of eye drops also could be used to attempt to control the fluctuations. Dr. Stewart agrees. "Prostaglandins absolutely are the best class that we know about for fluctuations based on Professor Konstas's and my findings. We found the level of fluctuation to be the best with Travatan [3.2 mmHg], and then Lumigan [3.5 mmHg]. We had the most experience with Xalatan [range 3.8 to 4.4 mmHg]," he says. .... Peak IOP still most often occurs during the nocturnal period. See http://www.revophth.com/content/d/cover_focus/i/1292/c/24893/).
- The diurnal curves are quite different between individuals, but consistent by person (across several tests). Some people have peaks first thing in the morning like I do (which I think is the most common time to peak), and others have peaks at 2pm in the afternoon, etc.
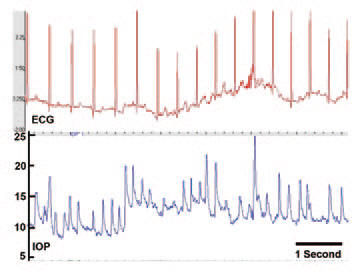
Why they can't seem to measure the same pressure twice in a row on a Reichert or Goldmann tonometer (or any other tonometer):
Screen capture of approximately six seconds of the continuous IOP and ECG signal from an awake, unrestrained nonhuman primate implanted with Dr. Downs’ IOP telemetry sensor. Note that the high-frequency fluctuations in IOP are due to saccades (eye movement) and blinks. But even without the blinks, you can see that the trough IOP pressure can vary from 8 to 14 over a 3 second period!!! This is why they always get different readings when they measure your IOP, no matter how accurate the tonometer. This is from The question of IOP.
This also explains why a Reichert 7CR measured me at 14 and 10, just seconds apart. I thought the machine was bad. But if you put a Reichert on a non-human "test eye," the readings are accurate to within .1mmHg.
There is also a waveform score that tells you how confident the machine was that it got a valid reading. Generally scores over 6 will give very reliable measurements. Most all self-tonometers lack this important feature (and it's really important since bad technique can give you bad measurements for most tonometers).
The Reichert air puff interval is 20 msec, and the inward/outward measurement of the 3mm diameter circle of the cornea is within 10 msec.
So the Reichert gives essentially an "instantaneous" measurement. So you can't take just a single snapshot on the Reichert because you might get unlucky and catch an IOP spike due to blinking, movement, fluctuations from the ocular pulse, and real variations in the trough IOP level that are constantly happening even over a time interval as short as a few seconds.
So looking at the graph above, that explains the wide range of IOP readings . Also, the Reichert is the only non-invasive technique I am aware of that can properly adjust for cornea properties (of which thickness is just one property) to tell you your true IOP. This is important because you cannot use cornea thickness to "correct" a Goldmann measurement because even the direction of the change depends on the dynamics of your cornea. So a thicker cornea might cause your Goldmann reading to be higher or lower. The only way to know is to use the Reichert on your eye and look at the IOPcc measurement. In general, Goldmann is really no longer state of the art, but physicians' habits are really hard to break. Note: you could argue that the "true" IOP doesn't matter since your cornea tends not to change so the Goldmann IOP is just fine as a relative measure of IOP.
Simplest IOP number when using a Reichert is to use the 3 puff average score. It looks at the "score" (not the IOP value) to determine weighting of the measurement.
If you are using a Reichert, because the peaks are really short and can vary a lot, it seems to me it would be much better to measure the lowest IOP value taken over several measurements (as long as the "score" is high so it is a good measurement), rather than just a single or mean value. This is because a trough measurement is much easier to take (and more meaningful) since it is sitting in the trough for most of the time of a measurement.
Why complement cascade is a very interesting pathway
However, in the mouse model of glaucoma, which very very closely if not identically models human glaucoma (which is quite nice because many animal models for other diseases do not mimic the human disease), one can watch progress loss of optic nerve axons over the 1 to 2 year course of the disease. In this model, the loss of optic nerve axons very closely tracks the death of retinal ganglion cells.
In case of interest, in this model, death of retinal ganglion cells starts to happen around 10 months of age however we have shown that complement activation at their synapses has already begun by 2 months of age and that massive loss of synapses is occurring already at 3 to 4 months of age (we have so far only looked at the synapse onto the retinal ganglion cells within the retina, but we presume the same thing is happening at the retinal ganglion cell synapses within the brain). This suggests that axons are ultimately lost because the synapses have degenerated. In fact, we showed in this mouse model by measuring how much optic nerve axon loss had occurred that by 11 months of age, the majority of control mice (with a normal complement system), had either moderate or severe glaucoma (optic nerve axon loss) whereas in complement knockout mice (that lacked the complement protein C1q), almost none had any evidence of glaucoma at all (we published this a couple of years ago). Blocking the complement system is profoundly neuroprotective for glaucoma. I hope that Calkins target works as well, but so far no other manipulation has come close to having such a profound effect, even the Wlds mutation which is a powerful promoter of axon survival
Advice I've received
-
first in regard to your eye problem:I don’t think there is much in non-IOP (intraocular pressure) clinical trials yet, but there are a number of compounds moving closer. I have participated in a few trial design advisory boards. Bob Weinreb (my chair) is very well-connected and will be able to keep Steve in the loop too. What the field really needs is a group to fund bringing candidates from the lab to the clinic. As for the ischemia from hyperviscosity, as you know that’s a principle hypothesis for etiology. There are some papers from China on hyperviscosity association with glaucoma but I don’t think that’s gone very far in research.
-
in regard to Calkins drug target, in regard to your visual fields: That kind of thing just needs to be tried. But looking at his visual fields I wouldn’t recommend he be the first guinea pig on any of these. Relatively speaking he is in decent shape, although I appreciate that if his functional vision is greatly impacted he might be motivated to try things...
Sleep study 12/8/13
The operated on eye never got above 25, where as the unoperated eye hit 29. So great news there.
The biggest delta between the eyes was sleeping on right side. Left eye was 21, right eye was 26
Sp02 no diff with and without cpap, HOWEVER, spo2 got down to 95 but don’t know for how long. John would have to chart it. That was the big surprise, but in general
CPAP: no diff. you can’t really argue it raised pressures. We did some tests at the start and there was no difference when I was under pressure or not on these tests, nor did the overall sleep study give pressures that we statistically significant lower. It clearly didn’t make things worse. If anything it made it better.
Sleep study 8/17/13
See sleep data. Note: They wake you up to take the measurements. For IOP, they use a Reichert pneumatonometer since it can measure while you are in the supine position.
Pressure in OS varied from 12.5 at 6:30pm (sitting) to 30.5 at 6:30am supine. So that is a huge variation.
Diurnal Variation in IOP found that Risk of disease progression within 5 years was six times higher for patients who had a diurnal IOP range of 5.4 mm Hg than for those with a diurnal IOP range of 3.1 mm Hg.
Comments from a doctor:
Independently of any other consideration, he should most definitely do a much better job of controlling his systolic blood pressure. It’s anomalously high for his age, especially when he’s supine-&-asleep! He should never tolerate a systolic pressure in excess of 120 mm-Hg under any conditions other than vigorous exercise, using an angiotensin receptor blocker (I recommend the modern type II flavor, e.g., valsartan-taken-daily) for long-term control, and a fast-acting peripheral vascular resistance-reducer, e.g., nicardipine-as-needed, for suppressing few-hour-scale transients-when-observed.
I predict a dramatic improvement in nocturnal regulation of intraocular pressure if he steadfastly controls his systolic pressure. [He’ll also do his cerebral and renal capillary beds a huge favor by doing so – they age linearly more rapidly as the mean pulse pressure climbs above a threshold of ~95 mm-Hg and, while his generally-good diastolic BP ‘helps’, it can’t save these crucial tissues from the ravages of his excessive systolic BP.] His intraocular pressure appears to be exceptionally sensitive to local blood pressure, as gauged by supine-vs.-sitting nocturnal measurements.
He may also benefit from seeing a cardiologist re getting active medical management of his nocturnal pulse rate, which seems quite high for his age and nominal health. Reducing it below ~80 BPM likely will help control nocturnal IOP to some degree, though less so than improved systolic BP control.
However, the optimal level of BP at night for glaucoma is not known. Lowering BP will have an imperceptible effect on IOP.
Trabectome surgery 8/26/13
Had trabectome surgery in my left eye. Next morning, IOP was 9 (at 8:30am). 1 week later it is 16 (at 2pm).
Medications: Prednisolone acetate is 1/hr then once every 2 hours starting on Friday. SHAKE it before use. Pilocarpine and zymaxid are to be used 4X/day (wake, noon, dinner, before bed).
Other: Protect eye with glasses during the day. At nigh, need to sleep with plastic eye protection patch for 2 weeks after surgery. Do NOT get the eye wet (lots of bad stuff in water).. Pinching is a good idea. Use dry tissue to clean debris around the eye. Minimize lifting and straining. Keep head above your heart at all times. sleep so that left eye is elevated. Next appt is 1 week out. No golf, situps, etc. Minimize nose blow, valsalva, etc. No sleep study for 3 months after eye operation. the feeling that there is something in my eye will go away in 2 days (it did). Because there is a hole in my eye that hasn't fully healed, it may NOT be bacteria resistant so that's why need to keep bacteria from the eye which is why water is a no no (it has tons of bacteria). Vision will get progressively better. By friday, left eye was slightly less sharp than right eye.
Safest way to clean all the junk around the eye and on eyelid in the first two weeks after surgery: get sterile qtips saturated with sterile saline.
Side effects of pilocarpine: head and brow ache: can take tylenol. It will also make your pupil small.
Reichert 9/4/13
Not designed for self tonometry, but they've sold over 200 that were
purchased by glaucoma patients for use at home. You have to reach around to hit the button.
It won't show you each result indpendently. If take three measures, it will
either average them or take the highest, whereas you really want the lowest so
need to clear it between each measurement. So easiest to set up a mirror so you
can see what is going on.
Low confidence score= you are moving your eye too much or not straight up and down. But anything above 6 is going to be pretty accurate. If you keep you eye steady, you can get up to 9.5 score.
If you do single measurements, the Reichert will remember a maximum of 3 measurements with waveform scores that are within 1 unit of each other. So the new measurement goes in only if has a higher "score" than the 3 that are there. Exception: if you hit the 3 puffs button, it clears all 3 scores when you hit the button.
So if you are using the single button alone, or after you hit the 3 puff button (and before you hit the next 3 puff button), it works like this:
1) Take measurements
Maximum number of measurements in memory is 3
2) The measurement with highest Waveform Score (WS) is identified internally by the software
3) All measurement(s) with a WS within 1.0 of Maximum WS, will be averaged to be displayed as IOPg, IOPcc & WS
4) After three measurements have been taken, a new measurement will replace the lowest waveform score measurement and steps 2 & 3 are repeated.
Super low IOP 1/14/2014
Measured a new low at 11pm last night: 7.5 OS 9.3 OD
My day was unusual for many reasons: late lunch, late dinner, ate at places I rarely eat at.
8:30am: Breakfast at Buck's restaurant: a filoli omelette (healthy version) with salsa, english muffin, and OJ
I starved at a meeting and finally went for a LATE Lunch at 3pm a new Chinese place in the mall I've never eaten at (it used to be Thai Orchid). I ordered the miso soup, and the tomato beef lunch special.
Dinner when I got home at 10pm was chicken and potatoes with Jack Daniels Master BBQ sauce on the chicken and fresh squeezed OJ to drink.
The only medication I took all day was Alphagan-P at 4pm (from a previously unopened bottle) and Lumigan the night before. So it wasn't the drugs.
Without the home Reichert, I never would have discovered this very interesting anomaly.
I repeated the measurement just to be sure and got 8.0 and 9.9. Stunning.
Just to be sure the machine wasn't broken, I then measured at 5:30am the next morning, immediately after hopping out of bed: 18.2 and 15.6
It appears that Something VERY interesting just happened.
IOP Test results
IOP before I started having problems with the field loss
2000: 14,14
2001: 15, 15
2002: 16,15
2004: 15, 17
2005: 14, 14
2006: 15, 13
2007: 14, 14
4/1/08: 13, 16
5/20/09: 13, 14
8/18/10: 14, 15My visual fields and IOP measurements
Kirsch glaucoma test data (everything I have)
My history
| Date | Event |
| 1980 | Probably developed sleep apnea around this time, but never knew it. Snoring has gotten worse over time. |
| 5/10/07 | Diagnosed with Waldenstrom's Macroglobulinemia. Kirsch Waldenstrom's Macroglobulinemia Diary is diary of drugs used and blood tests. |
| 9/11/07 | Surgery to remove appendix in Boston, MA. It was caught early, doing a routine medical exam. I was lucky. |
| 2/11/08 | High IgM event caused by Rituxan infusion to treat my Waldenstrom's. |
| 4/1/08 | Visual fields test at my annual eye exam shows vision loss in left eye. Doc thinks glaucoma so refers me to Tayeri. |
| 4/30/08 | Saw Tayeri for consultation because my regular eye doctor (Gary Stocker) noticed abnormality in visual fields which was likely caused by the high IgM event. |
| 1/28/09 | Started enzastaurin trial for my WM |
| 2/12/10 | Started LBH trial in Boston. Moved to Denver 10/26/10. |
| 12/3/10 | Surgery to remove gall bladder due to gallstones. I am now a shadow of my former self...no appendix, no gallbladder. Will my wife still love me? |
| 03/07/11 | Have had sleep apnea for a while, possibly a decade or more. Started using CPAP religiously due to the Respironics System One which is a fantastic improvement to the CPAP I used to have. So since CPAP added very late in the game, it is likely not the cause of my glaucoma. |
| 6/10/13 | Tayeri said my vision progressing too fast and I need surgery to get the pressures under 10. Saw Iwach. Changed medications to Zioptan, Simbrinza, Timolol |
| 6/12/13 | Iwach did SLT surgery on my left eye. |
| 6/27/13 | Pressure check at Iwach two-weeks post-surgery 10, 12 (left, right) |
| 7/31/13 | Saw Weinreb. Pressures back at 14 to 16 on Iwach's drugs. Weinreb told me to switch to his set of drugs which I did same day. |
| 8/5/13 | Shared my data with Marty Wax. He thinks the WM is the 800 lb Gorilla in my case. |
| 8/17/13 | Sleep study. Pressure in OS varied from 12.5 at 6:30pm (sitting) to 30.5 at 6:30am supine. See sleep data. My systolic pressure is pretty high at night (150 which is well above the 120 it should be) and my heartrate is high as well (should be below 80 bpm) |
| 8/26/13 | Tabectome surgery in OS. IOP in OS is 9 the next morning. Will it last? We shall see. |
Visual fields loss in left eye over time
| Date | Visual Fields (OD) | Visual Fields (OS) | Medications prior to this measurement |
| 12/7/09 | 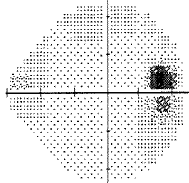 |
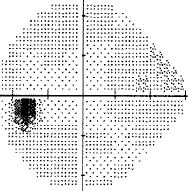 |
Xalatan left eye at night |
| 5/10/10 | 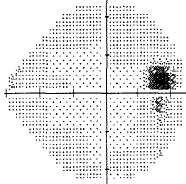 |
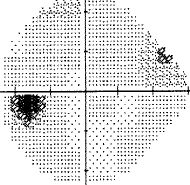 |
No medications for 6 months before this test |
| 5/12/11 |  |
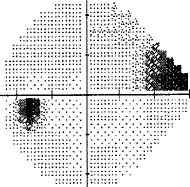 |
No medications for approximately 1.5 years prior |
| 4/30/12 |  |
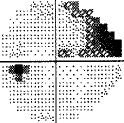 |
In the year prior, [Brimonidine, Combigan, none] given during this period (except for a 2 month period on Xalatan). These drugs have no effect at night (execept Xalatan). So nighttime pressures for 1 year were not treated except for a 2 month period. |
| 5/30/13 | 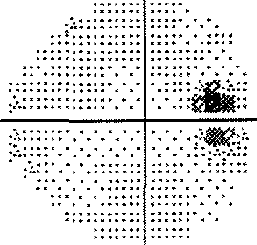 |
 |
Combigan (which has no effect at night) |
| 6/10/13 | 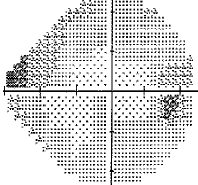 |
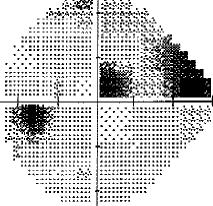 |
different drugs (some of which have effect at night) |
6/10/13 Cirrus OCT RNFL
| Date | RNFL thickness (OD) | RNFL thickness (OS) |
| 6/10/13 | 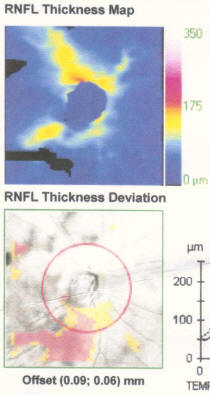 |
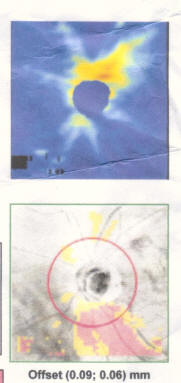 |
Medications
| Date | Pressure | Eye Medications | Cancer medications |
| 9/10/08 (Tayeri) | 13 | Xalatan OS qhs | Rituxan |
| 12/7/09 (Tayeri) | 17 | None | Enzastaurin |
| 5/25/11 (Tayeri) | 15 | Xalatan OU QHS | LBH-589 (Panobinostat): MWF |
| 8/4/11 (Tayeri) | 15 | Brimonidine OU bid | LBH-589 |
| 1/5/12 (Tayeri) | 15 | Combigan ou | LBH-589 |
| 6/10/13 (Iwach) | 16 |
Zioptan (before bed) |
LBH-589 |
| 7/31/13 (Weinreb) | 14 |
Latanoprost .005% (green top): at bedtime |
LBH-589 |
Medication comments
| Medication | Type | Notes |
|
Xalatan (Latanoprost) Zioptan (Tafluprost ophthalmic solution) Travatan (Travaprost) Lumigan (Bimatoprost)
|
Prostaglandin analog |
Dosed once a day, typically at night because that way
you don't see the red eye effect. These drugs have peak effect at 8 to 12 hours after instillation so timed to have max effect when your IOP peaks. They last a long time (a very flat efficacy curve and it still has impact on day 2), and they work at night. 1/8 patients respond much better to Lumigan, but side effects are 2 to 3 times more likely with Lumigan (see Prostaglandin analog trio equally effective in lowering IOP) which is why they lowered the formulation from .03 to .01%. Individual patients may respond differently to drugs within this class so you really should try and see what works best for you. For example, Lumigan .01% monotherapy in one patient lowered pressure 7mm more than Travatan plus Alphagan! In some patients, Lumigan is very good at controlling morning peaks which can be critically important (e.g., in people like me). So this is why it is important to try different drugs to see how they work for you. The expected IOP drop from baseline averages about 30% with once-daily dosing. Reduction in peak IOP: bimatoprost (33%), latanoprost (31%), travoprost (31%) and timolol (27%). Reduction in trough IOP: travoprost (31%), bimatoprost (28%), latanoprost (28%) and timolol (26%). Travoprost caused significantly more ocular hyperemia and eyelash changes than timolol or latanoprost and was equivalent to bimatoprost for these events Bimatoprost may decrease IOP slightly more than latanoprost and travoprost, however the clinical significance of this difference is not clear Side effects such as hyperemia, ocular pruritus, and eyelash growth are reported to occur more often with bimatoprost Doctors often prescribe prostaglandins to treat open-angle glaucoma. These eyedrops increase the outflow of the fluid in your eye (aqueous humor) and reduce pressure in your eye. Examples include latanoprost (Xalatan) and bimatoprost (Lumigan). Possible side effects include mild reddening and stinging of the eyes and darkening of the iris, changes in the pigment of the eyelid skin and blurred vision. Because these medications are fairly new to the market, complete, long term side effects are yet to be documented, and in fact, there is a newer side effect known as Prostaglandin-Associated Periorbitopathy See Ophthalmic Prostaglandins Review for detailed comparison. Also New drug Travatan shows excellent IOP lowering results in clinical trials |
| Alphagan-P (Brimonidine tartrate) | Alpha-adrenergic agonist |
Unlike Prostaglandins which have a pretty flat response curve (long half life but 50% effective at night), Brimonidine peaks in 1 to 4 hours with a half life of 3 hours and has minimal impact at night. These drugs, like beta blockers, do NOT work at NIGHT so it is stupid to take them 3X/day like on the label. This is why smart doctors tell you to take it in the morning, then 8 hours later. Max two doses/day. These medications reduce the production of aqueous humor and increase outflow of the fluid in your eye. Examples include apraclonidine (Iopidine) and brimonidine (Alphagan). Possible side effects include irregular heart rate, high blood pressure, fatigue, red, itchy or swollen eyes, and dry mouth.Pinch your nose when taking to reduce potential for CNS systemic effects. Note that brimonidine is said to have neuro-protective effects as well |
| Azopt (Brinzolamide ophthalmic suspension) | Carbonic anhydrase inhibitor | This has a relatively short effectiveness profile,
peaking at around 4-6 hours. They work at night as well as during the
daytime. Because of the short effectiveness profile, it is prescribed
for use 3 times a day.
It may not lower IOP in some patients. Experts say that "the best options [for lowering and stabilizing IOP] would be the prostaglandin analogs and carbonic anhydrase inhibitors" (see "The Question of IOP" in June 2011 issue of AAO EyeNet). These medications may reduce the production of fluid in your eye. Examples include dorzolamide (Trusopt) and brinzolamide (Azopt). Possible side effects include frequent urination and a tingling sensation in the fingers and toes. Side effects in drop form includes stinging, burning, eye discomfort. In pill form, there can be paresthesias, upset stomach, memory problems, depression and frequent urination. |
| Timolol Betaxolol |
beta blocker aka beta adrenergic blocker | Like alpha-adrenergic
agonists, these drugs do not work at night. Timolol can lower your blood pressure which (if you believe the OPP impacts glaucoma progression) is a really bad thing if you have glaucoma. Betaxolol lowers IOP without affective blood pressure much. One patient consistently dropped 22 points (systolic) and 15 points (diastolic) on Timolol. That can have a huge impact on glaucoma progression. When that patient switched to Lumigan, he went from a rapid progression to no progression. This was likely due to two important factors: Lumigan provides 24 hour IOP lowering and Lumigan does not lower your blood pressure. On the bright side, Timolol can lower your IOP during the daytime. One person went from 30 to 16 in just 24 hours. Ask your physician why he is doing this if this is one of your medications and if you still do it, monitor your blood pressure before and after. |
| Combigan | combination | Combigan (brimonidine and timolol) when prescribed as a
mono therapy for glaucoma is puzzling to me. There is good evidence that
most of the damage is done at night and both of these drugs in combigan
do NOT work at night.
The drug got FDA approval because it works in the day time, but the FDA hasn't figured out yet that IOP is highest at night. There is no point in taking this drug at night because there is no effect. You should not assume that just because a drug got FDA approval, it is a good drug to prescribe. One world expert in glaucoma I consulted pointed out that there are a lot of these glaucoma combination drugs that makes absolutely no sense and he never prescribes them. For most patients (whose IOP is highest at night), taking this drug may be worse than taking a placebo because not only is it ineffective at night, but the timolol can lower your blood pressure which is a risk factor for progression. I highly urge anyone who has been prescribed this drug as a monotherapy to ask their doctor for peer reviewed papers showing this combination works at night because just the opposite is true (there are papers showing it doesn't). I was prescribed this drug as a monotherapy and some of the worst progression I had was when I was on this drug. |
| Simbrinza | combo | Azopt + brimonidine. This isn't nearly as bad a combo as
combigan and saves time, but the brimonidine on the third daily dose is
pretty much wasted (since tiny impact at night) so you end up overdosing for
nothing.
Also, the .2% brimonidine in Simbrinza has higher allergy rate and more redness than the standard 0.1%. Moreover the 0.2% does not offer any advantage in IOP-lowering vs. .1%. So like Combigan, this is a drug that you really have to wonder why physicians prescribe it (the only reason I can think of would be for patients who have a compliance problem). If you still end up using it, do it for Morning and afternoon only. But for just before bed, you should do a Prostaglandin and Azopt. |
How to apply eye drops
http://www.fiteyes.com/home/glaucoma-eye-medications
DPP
diastolic OPP (DOPP)=diastolic BP-IOP. Under 50 is risky.
| Date | IOP (OS) | SYS | DIA | DOPP |
| 10/26/2010 | 19 | 104 | 66 | 47 |
| 1/18/2011 | 20 | 100 | 60 | 40 |
| 4/12/2011 | 22 | 140 | 80 | 58 |
| 7/7/2011 | 15 | 90 | 64 | 49 |
| 9/29/2011 | 15 | 126 | 82 | 67 |
| 12/15/2011 | 18 | 114 | 68 | 50 |
| 3/14/2012 | 15 | 130 | 78 | 63 |
| 6/6/2012 | 16 | 130 | 72 | 56 |
| 8/30/2012 | 17 | 116 | 68 | 51 |
| 11/28/2012 | 15 | 130 | 84 | 69 |
| 2/14/2013 | 16 | 120 | 70 | 54 |
| 5/9/2013 | 20 | 110 | 68 | 48 |
| 8/8/2013 | 14 | 118 | 66 | 52 |
Useful reading
Agreement and reliability of candidate tonometers for measuring intraocular pressure Shows that non-contact (air puff) tonometers agree within 0.28mmHg with GAT. This is a surprise to most physicians.
OCT measurement consistency: this is the key. It indicates you can get very stable numbers if the system is set up right. This is absolutely required in order to see the small changes that happen each day for someone rapidly progressing. It also suggests that if a reference image is made part of the system, you should be able to determine before the scan if the image is lined up and in focus. Done right, you can get <1um standard deviation between scans. So there is still a question about the reproducibility of dirunal data...should you average over the entire day? Should you take all samples at a certain time of the day?
Distribution of Ocular Perfusion Pressure and Its Relationship with Open-Angle Glaucoma: The Singapore Malay Eye Study. Mean ocular perfusion pressure (MOPP) = ⅔(mean arterial pressure − IOP), where mean arterial pressure (MAP) = DBP + ⅓(SBP − DBP), systolic perfusion pressure (SPP) = SBP − IOP, and diastolic perfusion pressure (DPP) = DBP − IOP. Low DBP, low MOPP, and low DPP are independent risk factors for OAG.
Is Normal Tension Glaucoma a Clinically Distinct Entity? Talks about CSF and blood pressure
Lumigan articles:
- http://medicaidprovider.hhs.mt.gov/pdf/lumigandossier.doc
- http://www.eyeworld.org/ewsupplementarticle.php?id=17
Triggerfish shows 24 hour IOP fluctuations
Cup to disk images and explanation
OPP article “In our study, we found that both ends of the blood pressure spectrum are damaging to the optic nerve.”
WALDENSTROM AND THE EYE by Dr. Maureen Hanley
· Normal-pressure glaucoma may be autoimmune neuropathy. Scerra, Chet // Ophthalmology Times;6/1/2003, Vol. 28 Issue 11, p16
Examines the relationship between the immune system and glaucoma. Evidence that normal pressure glaucoma (NPG) may represent an autoimmune neuropathy; Role played by the immune system in neuronal death among NPG patients; Hypothesis on the association of neurodegenerative processes in causing...
http://www.optometricmanagement.com/articleviewer.aspx?articleid=71619
The expected IOP drop from baseline averages about 30% with once-daily dosing.
Evening dosing is preferred as the onset of IOP lowering activity for
prostaglandin analogues is slow with peak effect at 8 to 12 hours after
instillation. Compare this with the two-hour peak efficacy of the beta-blockers,
for example.
http://www.jaypeejournals.com/eJournals/ShowText.aspx?ID=2030&Type=FREE&TYP=TOP&IN=_eJournals/images/JPLOGO.gif&IID=171&isPDF=YES
The recommended dosing regimen for Latanoprost, Travoprost and Bimatoprost is
once daily topical application preferably in the evening to reduce the early
morning dirunal spike. (p. 16)
http://emedicine.medscape.com/article/1206147-medication#7
IOP should be rechecked
at the drug's peak and trough times to see if the target IOP has been reached
and is maintained throughout the day.
http://www.ophthalmologymanagement.com/articleviewer.aspx?articleID=108143
From the beginning, OCT represented a trend-setting collaboration between engineering and medicine that dramatically improved patient care. The first publication on this innovative imaging technology appeared in Science in November 1991,1 following 10 years of work by Carmen A. Puliafito, MD, MBA, at Harvard Medical School, and James G. Fujimoto, professor of electrical engineering at the Massachusetts Institute of Technology. The goal of their work was to use short pulse lasers to measure ocular structures.
The inferotemporal (7 o’clock) sector was the most frequent location that showed progression, suggesting that this location is not only important in discriminating glaucomatous from healthy eyes10,11,26 but it should also play an important role in detecting glaucomatous progression.
For the global RNFL thickness, mean rate of change was −0.72 μm/year for progressors and 0.14 μm/year for non-progressors. The rates of change were widely variable among the eyes.
The ganglion cell complex is composed of the macular nerve fiber layer, ganglion cell layer, and inner plexiform layer. Assessment of these layers has been shown to have an improved ability to detect glaucoma compared with using full retinal thickness. Ganglion cell complex diagnostic accuracy for detecting glaucoma has been shown to be similar to that of peripapillary RNFL thickness,48–52 making it potentially valuable for monitoring glaucoma progression.
New Cirrus OCT 6.0 features Ganglion Cell OU Analysis: Macular Cube 512x128.
Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome.
http://willsglaucoma.org/some-medications-may-harm-glaucoma-patients Talks about use of medications like cortisones (post surgery), drugs that lower blood pressure (like Timolol), and drugs that dilate the pupil.
http://willsglaucoma.org You can search physician/patient q&a conversations. Very helpful site.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771773/ study re: how fast retinal thickness (independent of glaucoma) decreases each year with age
http://www.ncbi.nlm.nih.gov/pubmed/21478200 Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point
http://www.ncbi.nlm.nih.gov/pubmed/20853266 Intrasession, intersession, and interexaminer variabilities of retinal nerve fiber layer measurements with spectral-domain OCT
http://www.ncbi.nlm.nih.gov/pubmed/10511024
http://www.ncbi.nlm.nih.gov/pubmed/10070522
http://www.ncbi.nlm.nih.gov/pubmed/9164420
http://www.ncbi.nlm.nih.gov/pubmed/7639300
Comments from readers
Thanks, Rich. This man must have a lot of free time. He did an excellent job of reviewing the topic. Some of the auto-immune and other possible alternate therapies are under investigation and not yet accepted as therapy. He did a nice summary and I agree mainly on three points.
a) most ophths do not do nocturnal BP (I have for many years)
b) almost none of us do noctural intraocular pressures.The instruments are too expensive for patients and are hard to loan out. Patients get confused and breakage is a problem. I'll bet going to Bob Weinreb's sleep lab cost a fortune and probably isn't covered.
c) I agree that most ophths don't appreciate the 24h effectiveness of these drugs.
A nice piece by a smart man.